 What Is ICP Heavy Metal Analysis?
What Is ICP Heavy Metal Analysis?
Heavy metals, including arsenic (As), cadmium (Cd), chromium (Cr), lead (PB) and mercury (Hg) occur naturally in nature, and in low concentrations pose little health risk. When concentrations increase however, their presence can be toxic to plants, animals and humans. Because they are systemic toxins, routine testing is used to determine their presence and concentration. The United States Environmental Protection Agency (U.S. EPA), and the International Agency for Research on Cancer (IARC), classifies these metals as either “known” or “probable” human carcinogens.1
Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) is an analytical technique used to identify elements in samples, consequently it is well-suited for the detection of certain heavy metals. ICP-OES is also known as ICP-AES (Inductively Coupled Plasma Atomic Emission Spectrometry).
ICP-OES (and ICP-AES) use an extremely hot argon plasma to excite the atoms in a liquid sample, promoting them from a ground state to an excited state. As the element returns from the excited state to the ground state, light is emitted at specific emission line wavelengths, which are then directed to the system’s spectrometer for measurement by a highly-sensitive camera (also called a detector).
An ICP-OES instrument consists of three primary components/systems: the sample introduction system, the inductively coupled plasma (ICP) from which the specialization derives its name, and the optical emission spectrometer that houses the light separating optics and detector.
The first component we will discuss in detail is the sample introduction system that transports the liquid sample to the plasma torch. The sample introduction system consists of the peristaltic pump and tubing, spray chamber with nebulizer, and torch with injector tube where the plasma is formed.
The nebulizer is installed in the spray chamber and converts the liquid sample from the peristaltic pump to a fine aerosol. This sample aerosol passes through the spray chamber where large droplets are removed via a drain. The sample aerosol is then directed to the torch’s injector tube where it meets the inductively coupled plasma.
The plasma, the second component of an ICP instrument, is the source of energy used to convert the sample analyte atoms in the aerosol from a ground state to an excited state. The plasma is created by passing an alternating current through a cooled induction copper coil where a magnetic field is created. Within the magnetic field, accelerated electrons move in a circular path, while a tangential flow of argon gas flows through the quartz torch. The collision of argon atoms and electrons forms an exceptionally hot, stable plasma discharge at the end of the quartz torch.
The argon plasma achieves temperatures in the order of 10,000 °K which is capable of exciting the analyte atom’s outer shell electrons to a higher energy level. When they eventually fall back down to the ground state, they emit light at specific wavelengths which are characteristic of the elements of interest.
Using a system of finely tuned optics, the image of the plasma (containing the samples wavelength emission line and light intensity information) is directed to the optical spectrometer, which is the third significant system of an ICP-OES instrument.
In the spectrometer, individual emission lines are separated from each other using a series of focusing optical components and a high-resolution dispersion grating. Finally, these emission lines are detected either simultaneously or sequentially with a photosensitive detector that converts light energy into electrical energy. A majority of ICP detectors are either charge-coupled detectors (CCD) or charge-injection detectors (CID). The electrical energy is then used to quantitate the elements present in the sample and their respective concentrations, using formula’s calculated by the instrument’s software.
Performing Heavy Metal Analysis Process
Due to concerns about heavy metal toxicity, laboratories worldwide perform heavy metals analysis by ICP-OES of sample matrices including drinking water, wastewater, soils/sludges, food, and others. Soils are often a common location for high concentrations of heavy metals, chemicals, and wastes as by-products of industrial and agricultural pollutants that take a significant amount of time to break down.2 Additionally, governing agencies such as the US FDA protect the safety of the public food supply through monitoring, testing and setting standards for metals in foods, animal feed and cosmetics.3
Detecting heavy metals in samples is typically performed using the following steps:
-
The sample is collected from the source to be tested. Samples may be acidified for preservation, depending on the sample type.
-
The collected sample is then sent to the laboratory for analysis by ICP-OES within a specified time-span (after which analytical accuracy will diminish).
-
Once the sample arrives at the laboratory, the date and time it is received is recorded.
-
Because ICPs analyze samples in liquid form, samples originating from solid matrices will require digestion/extraction. Using special solvents, acids and laboratory apparatus, the sample is then prepared for analysis.
-
Depending on the sample type and solvent/acid used for digestion, appropriate sample introduction components for the ICP are selected. Peristaltic pump tubing type, type of nebulizer and spray chamber design, and plasma torch injector tube diameter are all carefully chosen to optimize analysis and maintain resistance to the sample matrix.
-
Depending on the sample type to be analyzed, instrument operating conditions are carefully determined to optimize analysis. Operating parameters typically include: sample uptake rate (peristaltic pump speed), RF Power (current to the load coil), nebulizer pressure (to convert the sample to a liquid aerosol in the spray chamber) and coolant gas flow/ auxiliary gas flow to protect the torch from the plasma.
-
Once all these steps have been taken, the sample is analyzed for heavy metal content and a report of the analytical data is created.
Teledyne Leeman Labs’ ICP-OES Instruments for Heavy Metal Analysis
In 30 years of supplying inductively coupled plasma (ICP) Spectrometers, we've learned no one ICP-OES is ideal for all applications. All Teledyne Leeman Labs’ ICP-OES instruments are available in Radial, Axial or Dual-View Configurations, so the view that is best suited to a sample matrix can be chosen.
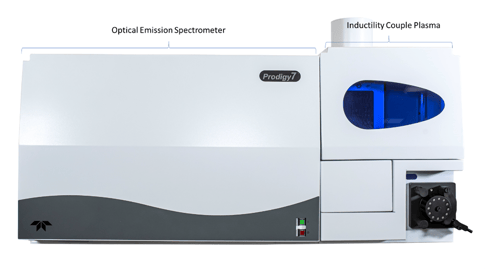
Prodigy7 ICP-OES
 The Prodigy7 is the summation of years of refinement, and has distinct advantages over other ICPs including: large format, advanced CMOS array detector for true simultaneous measurement; full wavelength coverage from 165 nm - 1100 nm; Full Spectral Access (FSA) feature that captures the entire wavelength spectrum in a single reading; twist-n-Lock auto-aligning sample introduction system; compact benchtop design; fast system startup; and reduced gas consumption.
The Prodigy7 is the summation of years of refinement, and has distinct advantages over other ICPs including: large format, advanced CMOS array detector for true simultaneous measurement; full wavelength coverage from 165 nm - 1100 nm; Full Spectral Access (FSA) feature that captures the entire wavelength spectrum in a single reading; twist-n-Lock auto-aligning sample introduction system; compact benchtop design; fast system startup; and reduced gas consumption.
ProdigyPlus ICP-OES
 The Prodigy Plus ICP combines the latest in solid-state detector technology with our advanced, high-dispersion Echelle spectrometer to provide a powerful ICP. The Prodigy Plus provides superb resolution, stability and detection limits for reliable results. Features include large format, CMOS-based, advanced, solid-state array detector; full wavelength coverage from 165 - 1100 nm (130 - 1100 nm with halogen option); full-frame imaging to capture the entire ICP spectrum at once; and application-specific sample introduction system options.
The Prodigy Plus ICP combines the latest in solid-state detector technology with our advanced, high-dispersion Echelle spectrometer to provide a powerful ICP. The Prodigy Plus provides superb resolution, stability and detection limits for reliable results. Features include large format, CMOS-based, advanced, solid-state array detector; full wavelength coverage from 165 - 1100 nm (130 - 1100 nm with halogen option); full-frame imaging to capture the entire ICP spectrum at once; and application-specific sample introduction system options.
FAQs
What can ICP-OES detect?
-
ICP-OES can handle a wide range of sample matrixes ranging from soil to drinking water, waste water, beverages, petroleum-based oil samples, and many others.
-
ICP-OES can detect a wide range of elements that include As, Se, K, Mg and others.
-
ICP-OES is useful to determine the type of elementals that may be present in samples.
For more information on Teledyne Leeman Labs ICP-OES products and applications visit: http://www.teledyneleemanlabs.com/products/icp-oes or click the button below to have someone contact you:
References:

