Factsheet and Understanding the Importance of Testing Mercury in Wastewater
 What is Wastewater and Why Treat it?
What is Wastewater and Why Treat it?
Wastewater is made of all ‘used’ water. Its composition ranges widely from grey water produced in homes and businesses to runoff into storm drains. It also includes sewage sludge and industrial process waste. Wastewater contains any number of substances including chemicals, soaps, food scraps, oils, and human waste.
Humans produce billions of gallons of wastewater and if left untreated the environment would be overwhelmed with contamination. Water must be treated before it is transferred back into the environment to protect ourselves and nature from pollution. Water needs to be clean for wildlife species that depend on water, beach and marsh habitat to survive. Treated, clean water also prevents disease and allows for scenic and recreational enjoyment of our waters for humans. Wastewater treatment should be a priority for maintaining a high quality of life for people and animals.1
Environment Canada provides some examples of pollutants that can be found in wastewater. The potentially harmful effects these substances can have on ecosystems and human health include:
- decaying organic matter and debris can use up the dissolved oxygen in a lake so fish and other aquatic biota cannot survive;
- excessive nutrients, such as phosphorus and nitrogen (including ammonia), can cause eutrophication, or over-fertilization of receiving waters, which can be toxic to aquatic organisms, promote excessive plant growth, reduce available oxygen, harm spawning grounds, alter habitat and lead to a decline in certain species;
- chlorine compounds and inorganic chloramines can be toxic to aquatic invertebrates, algae and fish;
- bacteria, viruses and disease-causing pathogens can pollute beaches and contaminate shellfish populations, leading to restrictions on human recreation, drinking water consumption and shellfish consumption;
- metals, such as mercury, lead, cadmium, chromium and arsenic can have acute and chronic toxic effects on species.
- other substances such as some pharmaceutical and personal care products, primarily entering the environment in wastewater effluents, may also pose threats to human health, aquatic life and wildlife.’2
Dangers of Mercury Pollution in Waters
Let’s take a deeper look into mercury contamination in wastewater. Mercury is a bio-accumulative, toxic element. It is found naturally and can also be released to the atmosphere, soil, and water from a variety of anthropogenic sources. With its high toxicity, ongoing mercury exposure in humans can lead to poor health outcomes such as tremors, muscle weakness, motor and speech impairment and a decline of cognitive abilities. The most common means by which people in the United States are exposed to mercury is by ingesting contaminated food and water.3 Mercury enters the food chain as bacteria in water and soil process inorganic mercury into methylmercury. Since animals accumulate methylmercury faster than they eliminate it, concentrations increase at each level of the food chain. This process is called biomagnification. The associated risk for humans and animals is determined by the form of mercury present, the duration and route of exposure, environmental conditions, and ecological factors that influence how mercury moves and changes in the environment.4 Monitoring and removing this toxin in wastewater is required to ensure environmental and human health.

Figure 1: Common loon feeding a crayfish to its chick.
Birds which eat fish and aquatic invertabrates accumulate the
mercury contained within their prey.
How to Remove Mercury from Industrial and Waste Waters
Wastewater treatment plants are essential for cleaning water before releasing it back into the environment. Water may be treated differently depending on the source. Surface waters typically require more processing than ground waters because they contain more particles, chemicals, and toxins. Common methods used to process the water are physical means like allowing solids to settle in ponds, chemical treatment like chlorine, and using UV light to kill organisms like bacteria.5 Special treatments are needed for removing mercury and heavy metals.
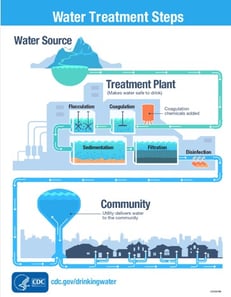
Figure 2: Water Treatment Steps: Coagulation, Flocculation, Sedimentation, Filtration, and Disinfection. https://www.cdc.gov/healthywater/drinking/public/water_treatment.html
While there are proven techniques for mercury removal from wastewater, each has pros and cons depending on the makeup of the water entering the wastewater treatment plant. Some factors to consider are pH, temperature, flow rate, volume, biological oxygen demand (BOD), and target mercury concentration.
There are three common wastewater treatment processes used for mercury removal: precipitation, adsorption, and membrane filtration.
Precipitation: This is the most common wastewater treatment for water containing mercury pollution. It is cost effective and simple to operate. First, a chemical is added to the stream which reacts with the dissolved solids. The precipitant can remove mercury in two different ways: by forming insoluble mercury compounds or by forming other solids that absorb the dissolved mercury. After the solid is formed, it can be separated by filtration. Some disadvantages of this wastewater treatment technique that it is often not sufficient to reduce mercury concentrations below accepted limits and it also creates a sludge. There are special disposal rules regarding sewage sludge and semi-solid hazardous materials which could be a burden on municipal wastewater treatment plants.
Adsorption: This is the second most common because it does not generate sludge and has a good selectivity for mercury and other metals. Usually a wastewater stream will pass through a bed of adsorbent media (like activated carbon or ion exchange resins) where dissolved contaminates are attracted and removed from the stream. Over time the media will become saturated and need to be replaced or restored. Replacement of the absorbent media is a significant expense but when the wastewater treatment plant is properly designed, adsorption has low operational costs compared to other removal technologies.
Membrane filtration: This mercury removal method passes the wastewater stream through a semi-permeable membrane which has pores which block large particle and allow smaller particles to pass. Nanofiltration and Reverse Osmosis have pore sizes small enough to block divalent cations like mercury. While this treatment type offers a high removal rate for mercury, it is vulnerable to flow rate issues and clogging.6
Finding the right combination of technologies will be different for each wastewater treatment plant. It is also worth noting that wastewater discharge standards are subject to change, and it is important to consider future compliance needs when investing in new technologies. Using a combination of these accepted wastewater management practices can safely remove mercury from polluted sources.
How Teledyne Leeman Labs Tests for Mercury in Wastewater
The US Environmental Protection Agency (EPA) sets the standard for acceptable mercury concentrations in drinking and wastewater. Currently approved chemical test methods include method 245.1, method 245.7, method 7470A, and method 1631, all of which provide guidance for the determination of mercury in various waters, via Cold Vapor Atomic Absorption (CVAA) or Cold Vapor Atomic Fluorescence (CVAF).7,8
Teledyne Leeman Labs provides atomic spectroscopy instrumentation based on US EPA, ISO, ASTM and other methods to environmental and water quality laboratories around the world. Below is a brief overview of our mercury analyzers for aqueous samples.
The QuickTrace® M-7600 Cold Vapor Atomic Absorption (CVAA) mercury analyzer design is based on the US EPA method 245.1 but provides the additional ability to analyze samples in the trace range of less than 1 ng/L. The applicable methods typically require sample digestion techniques that produce liquid samples with mercury present in the +2-valence state. The M-7600 easily achieves ultra-trace mercury detection limits of <0.5 ng/L and is an ideal analyzer for ultra-trace to sub-mg/L mercury quantitation in digested native solutions and raw industrial manufacturing materials. Capable of analyzing virtually any aqueous acidified sample, the QuickTrace® M-7600 was specifically designed for routine and research use in a variety of settings including environmental laboratories, industry and research institutes.

Figure 3: Teledyne Leeman Labs QuickTrace M-7600 CVAA Mercury Analysis System
The QuickTrace® M-8000 Cold Vapor Atomic Fluorescence (CVAF) mercury analyzer is based on methods US EPA 1631, US EPA 245.7 and other CVAF methodologies. It is ideal for ultra-trace to sub-mg/L mercury quantitation. Due to the high sensitivity of the M-8000, it easily achieves the ultra-trace detection limit of <0.05 ng/L total mercury that is demanded by customers following EPA method 1631. The QuickTrace® M-8000 is also versatile enough to analyze samples >400 µg/L without dilution in a research or industrial setting.
The Hydra IIAA mercury analyzer design is based on US EPA 245.1 It delivers the performance required to meet tightening regulatory demands and the productivity needed for laboratories to operate efficiently. Its parts-per-trillion Instrument Detection Limit (IDL) for total mercury, exceptional stability and unique over-range protection easily satisfy the most stringent quality control (QC) specifications. Its high capacity autosampler with extra-large CCV/CCB containers permits long periods of unattended operation.
Contact us for more information or visit our website- http://www.teledyneleemanlabs.com/products/mercury-analyzers
FAQs
- How does mercury get into lakes and streams?
Mercury makes in way into aquatic ecosystems by two main routes: source discharges (including manmade pollution, volcanoes, and geologic deposits breakdown) and atmospheric deposition (including rain and snow).
- How does mercury enter the food chain?
Bacteria in the environment can convert inorganic mercury to methylmercury through a metabolic process. Methylmercury is considerably more toxic and persistent than inorganic mercury. The bacteria accumulate methylmercury faster than they can excrete it so it bioaccumulates. When something comes along and eats the bacteria containing methylmercury, it is transferred to them. This is called biomagnification. Animals consume higher concentrations of mercury at each tropic level of the food
References
1 https://www.usgs.gov/special-topics/water-science-school/science/wastewater-treatment-water-use
2 https://www.canada.ca/en/environment-climate-change/services/wastewater/pollution.html
3 https://www.atsdr.cdc.gov/toxguides/toxguide-46.pdf
4 https://www2.usgs.gov/themes/factsheet/146-00/
5 https://www.cdc.gov/healthywater/drinking/public/water_treatment.html
6 https://samcotech.com/how-to-remove-mercury-from-your-industrial-water-and-wastewater/
7 https://www.epa.gov/cwa-methods/approved-cwa-chemical-test-methods
8 https://www.epa.gov/sites/default/files/2015-12/documents/7470a.pdf
