Mercury Testing in Water and Health Effects of Mercury in Drinking Water

How does mercury get into drinking water supplies? How do we know when our drinking water is polluted? How does mercury contamination impact human health? In this blog we will focus on the sources of mercury in the environment, discuss health risks associated with mercury exposure, examine water treatment for mercury, review existing regulation designed to monitor and mitigate mercury and highlight Teledyne Leeman Labs’ role in manufacturing analyzers that test for mercury in water samples.
Sources of Mercury
Mercury is extremely rare in comparison to other elements within the Earth’s crust. Although sequestered within the Earth, mercury can still pose a threat through absorption by animals and plants, or leaching into bodies of water. Mercury has been mined by some of the earliest civilizations including the Egyptians, Greeks, Romans and ancient Chinese, and has been employed by mankind in a variety of uses. While volcanoes, forest fires, cinnabar ore and fossil fuels are natural sources of mercury, a majority of global mercury emissions are produced by human activity. Mercury sources can be classified into two broad categories: naturally occurring and anthropogenic emissions. Mercury never leaves the environment, so mercury emissions recycle and change forms for thousands of years (there is uncertainty on its lifespan). From this cycle, the historical emissions join with modern emissions to form a “reservoir.”1
The historical mercury stored in soils and waters acts as a reservoir making atmospheric mercury concentrations higher than the amount resulting from modern emissions alone. Global inventory of mercury air emissions from anthropogenic sources was estimated to be 2220 tons in 2015. 60% of that total is reintroduced to the air from sources deposited into the soil and water. About 30% of the mercury emitted annually into the atmosphere is anthropogenic. The other 10% is from natural sources such as volcanic activity.
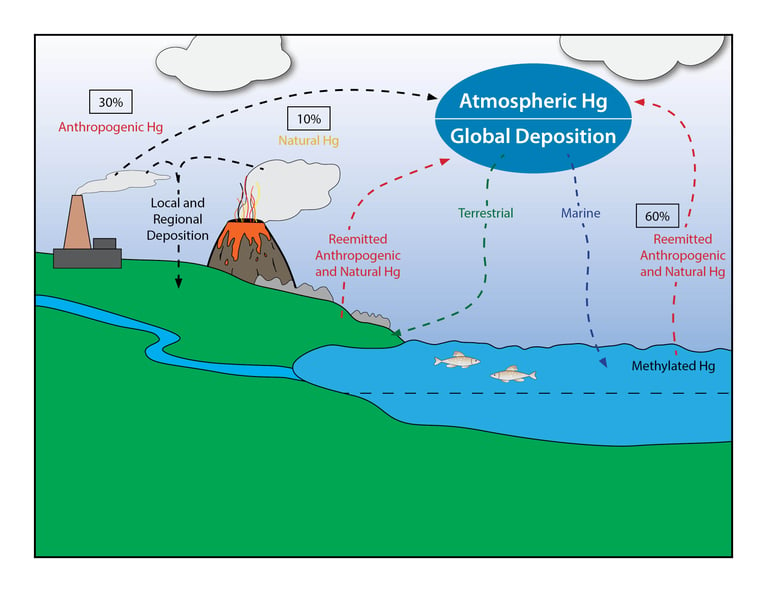
Figure 1: Simplified geochemical cycle of mercury (Hg). Recreated from USGS Fact Sheet FS-095-01: Mercury in U.S. Coal – Abundance, Distribution, and Modes of Occurrence by S.J.Tewalt, L.J. Bragg, and R.B. Finkelman, U.S. Department of the Interior, U.S. Geological Survey, September 2001,.2
Anthropogenic sources of mercury emissions can be divided into four large categories:
- Intentional use (e.g. landfills and product waste)
- Fuel combustion (e.g. powerplants and boilers)
- Industry sectors ( e.g. manufacture of metals, alkali, cement and nonferrous metal production)
- Artisanal and Small-scale Gold Mining (ASGM) 2
Globally, ASGM is the largest source of manmade mercury emission at 37.7%, followed by fuel combustion at 21%. The other two large emitters are non-ferrous metal production (15%) and cement production (11%). Once emitted, the mercury gases and particulates are transported globally and eventually deposited back to the earth by a process known as atmospheric deposition. Once deposited, the mercury collects in soil, bodies of water, sediments and surface water.3
When Does Mercury Become a Problem for Human Beings?
All forms of mercury can cause health problems, but the risks are determined by the dose, duration and the route of exposure. Elemental mercury is usually absorbed through inhalation of vapors. The most common means by which people in the United States are exposed to mercury is by ingesting contaminated food and water. Some populations are at a higher risk than others when it comes to mercury exposure, especially exposure to methylmercury contaminated fish.4 These groups include pregnant and nursing women, children, older adults and people who consume more fish than the average person.5
The CDC states the following Minimal Risk Levels (MRLs) for oral routes of mercury exposure:
- An acute-duration (≤14 days) oral provisional MRL of 2 µg Hg/kg/day was derived for inorganic mercury.
- An intermediate-duration (15–364 days) oral provisional MRL of 0.01 µg Hg/kg/day was derived for inorganic mercury.
- A chronic-duration (≥365 days) oral provisional MRL of 0.1 µg Hg/kg/day was derived for methylmercury.6
Mercury Health Risks
A wide variety of human health effects are associated with mercury exposure, some of which are irreversible. Exposure can lead to many neurological symptoms such as tremors, muscle weakness, motor and speech impairment and a decline of cognitive abilities. Other symptoms can include skin rashes, memory loss, sudden changes in mood, issues with vision and a feeling of “pins and needles” in hands and feet.
As mercury is distributed around the body, the highest concentration is found in the kidneys, leading to impairment of renal function. Additionally high blood pressure and changes to the immune system have been observed in humans and animals. It is also important to note that mercury can cross the placenta and has been found in umbilical cord blood and breast milk. Neurodevelopment and birth defects have been observed in humans with prenatal exposure to mercury compounds. Lastly, multiple forms of mercury have been classified as possible human carcinogens. When mercury exposure and poisoning is suspected, it is advised to not allow symptoms to persist without seeking medical treatment, as they may become permanent.9
Is Your Water Safe to Drink?
 If you suspect you have mercury contaminated water, there are a series of steps to take to determine the treatment process. The first of these steps is observational self-testing. Gather information about the water source used to supply your drinking water. Is it from a public water supply (controlled by the city) or a private water system (like a well on your property)? If it is from a public supplier, the EPA requires a Consumer Confidence Report be provided annually for most water systems. Are you located in an area known for high mercury content? Are you located near a coal processing facility or energy producer that uses coal? Are you close to any mines that use mercury? What about landfills or waste processing systems? All of these can be sources of mercury in the environment. Many drinking water contaminants can reach hazardous levels before a noticeable taste, odor or appearance indicates there is a problem. Do not wait for signs of mercury poisoning.
If you suspect you have mercury contaminated water, there are a series of steps to take to determine the treatment process. The first of these steps is observational self-testing. Gather information about the water source used to supply your drinking water. Is it from a public water supply (controlled by the city) or a private water system (like a well on your property)? If it is from a public supplier, the EPA requires a Consumer Confidence Report be provided annually for most water systems. Are you located in an area known for high mercury content? Are you located near a coal processing facility or energy producer that uses coal? Are you close to any mines that use mercury? What about landfills or waste processing systems? All of these can be sources of mercury in the environment. Many drinking water contaminants can reach hazardous levels before a noticeable taste, odor or appearance indicates there is a problem. Do not wait for signs of mercury poisoning.
Once you have gained some background on the possible contamination of your water supply, order an at home water quality testing kit. These test kits are usually rapid tests consisting of a color changing test strip and a chart to compare the result. Many include results for other heavy metals or characteristics like pH. It is important to note the detectable range on the test. If the at home test isn’t sensitive enough, it may not identify mercury in your drinking water. There are also at home test kits available to determine mercury levels in the human body if you would like to check yourself for heavy metal contamination. If you think you have had a chronic mercury exposure, it is very important to seek medical assistance as soon as possible.
If your screening test kit is positive for mercury, sending a water sample to an accredited water quality testing lab is the next step. Prepare your sample at home according to the lab’s directions and then send it to them. Laboratory testing provides assurance that your sample was analyzed by an expert. If the test results are positive for mercury in water, the next step is a certified water quality test and treatment.
Certified water testing is in a different category than sending a water sample to an environmental lab. Certified tests have a water professional come to your home and prepare the sample, thus establishing a chain of custody. Then an accredited lab certifies your results are of the highest accuracy. These types of test results can be used as valid documentation in legal cases, if needed. 9
Mercury Treatment
If test results show your drinking water is contaminated, take precautions immediately. As an interim solution, replace drinking water with bottled water or another safe, potable water source. Use this source for all drinking and cooking. Do not boil the contaminated water because this can volatilize the mercury in water. For a long-term solution, a water quality professional can suggest and install a water treatment system that is best for your unique water composition. Common treatment systems for mercury include activated carbon-based filters, reverse osmosis and other purification technologies. 9
Testing Drinking Water for Mercury
A significant amount of regulations and standards have been enacted by the US EPA, with a concerted effort to regulate and monitor mercury contamination and mitigate human health risks. There are three important regulations regarding mercury contamination, which cover not just water, but also air quality due to the cyclical nature of mercury:
-
1972- The Clean Water Act which adopted water quality standards for states. The standards specify mercury levels that “must be met to protect human health, fish and wildlife” and prohibit the discharge of mercury into waters without permit.10
-
1973- The Safe Drinking Water Act which set standards for public water systems, limiting mercury levels in drinking water. 11
-
2005- The EPA issued the Clean Air Mercury Rule “which creates performance standards and establishes permanent, declining caps on mercury emissions. The Clean Air Mercury Rule marks the first time EPA has ever regulated mercury emissions from coal-fired power plants.12
In the United States, the appropriate mercury testing technique for determination of mercury in drinking water is dictated by choosing the relevant, approved EPA method. Approved methods for analysis ensure that no erroneous or biased data is generated due to sample preparation, instrument settings and other analytical variables that could impact the accuracy of the test. Currently approved EPA methods include: method 245.1, method 245.7, method 7470A, and method 1631, all of which provide guidance for the determination of mercury in various waters, via Cold Vapor Atomic Absorption (CVAA) or Cold Vapor Atomic Fluorescence (CVAF).13
Our Expertise in Environmental Testing
 Teledyne Leeman Labs provides atomic spectroscopy instrumentation based on US EPA, ISO, ASTM and other methods to environmental and water quality laboratories around the world. Below is a brief overview of our mercury analyzers for aqueous samples.
Teledyne Leeman Labs provides atomic spectroscopy instrumentation based on US EPA, ISO, ASTM and other methods to environmental and water quality laboratories around the world. Below is a brief overview of our mercury analyzers for aqueous samples.
The QuickTrace® M-7600 Cold Vapor Atomic Absorption (CVAA) mercury analyzer easily achieves ultra-trace mercury detection limits of <0.5 ng/L, and is an ideal analyzer for ultra-trace to sub-mg/L mercury quantitation in digested native solutions and raw industrial manufacturing materials. Capable of analyzing virtually any aqueous acidified sample, the QuickTrace® M-7600 was specifically designed for routine and research use in a variety of settings including environmental laboratories, industry and research institutes.
The QuickTrace® M-8000 Cold Vapor Atomic Fluorescence (CVAF) mercury analyzer is ideal for ultra-trace to sub-mg/L mercury quantitation. Due to the high sensitivity of the M-8000, it easily achieves the ultra-trace detection limit of <0.05 ng/L total mercury that is demanded by customers following EPA method 1631. The QuickTrace® M-8000 is also versatile enough to analyze samples >400 µg/L without dilution in a research or industrial setting.
The Hydra IIAA mercury analyzer delivers the performance required to meet tightening regulatory demands and the productivity needed for laboratories to operate efficiently. Its parts-per-trillion Instrument Detection Limit (IDL) for total mercury, exceptional stability and unique over-range protection easily satisfy the most stringent quality control (QC) specifications. Its high-capacity autosampler with extra-large CCV/CCB containers permits long periods of unattended operation.
Contact us for more information or visit our website- http://www.teledyneleemanlabs.com/products/mercury-analyzers
References
- https://en.wikipedia.org/wiki/Mercury_(element)
- https://pubs.usgs.gov/fs/fs095-01/fs095-01.pdf
- https://www.epa.gov/international-cooperation/mercury-emissions-global-context
- https://wwwn.cdc.gov/TSP/MMG/MMGDetails.aspx?mmgid=106&toxid=24
- https://www.epa.gov/mercury/basic-information-about-mercury
- https://www.atsdr.cdc.gov/toxguides/toxguide-46.pdf
- https://www.consumerreports.org/cro/news/2014/08/how-to-reduce-your-mercury-exposure-from-seafood/index.htm#articleSections_articlesection_3
- https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations
- https://www.knowyourh2o.com/indoor-6/mercury
- https://www.epa.gov/laws-regulations/summary-clean-water-act#:~:text=(1972),quality%20standards%20for%20surface%20waters
- https://www.epa.gov/laws-regulations/summary-safe-drinking-water-act
- https://archive.epa.gov/mercuryrule/web/html/basic.html
- https://www.epa.gov/cwa-methods/approved-cwa-chemical-test-methods

