Do you have a backlog of solids and/or semisolid samples you need to analyze for mercury determination? Or are you a teaching laboratory needing an instrument to complete that proposal you wrote? Or a laboratory exploring options to enhance your portfolio? Well, I encourage you to look to Teledyne Leeman Labs for viable options.
Read MoreTeledyne Leeman Labs Blog
Usefulness of a Direct Combustion Mercury Analyzer
Posted by Jeff Forsberg on Jun 29, 2023 2:23:47 PM
Tags: mercury, mercury analysis
Run it, don’t pollute it — filter your mercury exhaust
Posted by Aaron Keller on Jun 15, 2023 11:43:46 AM
Mercury poisoning is a serious concern for those of us who directly work with mercury. Mercury is a heavy metal with many adverse side effects for people and the environment, even in relatively small amounts. That is why mercury analyzers are useful for testing where health concerns exist; you likely use one in your own lab if you’re reading this.
Read MoreTags: mercury, mercury analysis
We often get asked if there is an ideal photo multiplier tube (PMT) voltage for the QuickTrace® M-8000. The truth is the optimal value is unique to each instrument’s detector. It varies from instrument to instrument. During the initialization process, after connecting to the QuickTrace software, the M-8000 will select the voltage that is right for the PMT inside that system. This is the same process as clicking the Auto Select Voltage button in the Instrument Control menu. So, why is there another Set Volts option in that same menu?
Read MoreTags: Teledyne Leeman Labs, mercury, QuickTrace
We are in a battery recycling boom! A strong push from consumers for corporate responsibility in source origins and greener options overall has been felt by companies all over the world. Using recycled materials can be a cost-effective, logical choice for supporting Green Initiatives and boosting the bottom line.
Read MoreTags: mercury, Green Materials, Battery Recycling
Getting to Know Your QuickTrace® 7600 Hg Analyzer - Liquid flow: Pump Tubing (Part 3 of 3)
Posted by Aaron Keller on May 11, 2023 9:50:28 AM
This is the third in a three-part series on Getting to know your QuickTrace® — The effect of liquid flow.
To catch up on the other blogs in this series click the links below:
- Part one: Getting to Know your QuickTrace®: The Effect of Liquid Flow: Pump Rate
- Part two: Getting to Know Your QuickTrace® - The Effect of Liquid Flow: Importance of Capillary Action
Tags: mercury analysis, QuickTrace
Getting to Know Your QuickTrace® - The Effect of Liquid Flow: Importance of Capillary Action (Part 2 of 3)
Posted by Aaron Keller on May 2, 2023 3:58:24 PM
This is the second in a three-part series on the QuickTrace® .
Part one is linked here, “Getting to Know Your QuickTrace®: The Effect of Liquid Flow — Pump Rate"
The importance of the moment the liquid sample is introduced to the gas liquid separator (GLS) via the capillary cannot be understated. Read MoreTags: mercury analysis, QuickTrace
Getting to Know Your QuickTrace®: The Effect of Liquid Flow: Pump Rate (Part 1 of 3)
Posted by Aaron Keller on Apr 26, 2023 9:53:51 AM
There are always lots of factors when it comes to running any samples: concentrations present, liquid and gas flow rates, unit detection limits, to name a few. The liquid flow is highly important in the QuickTrace® M7600 and QuickTrace® M8000 due to the sample and reagents being introduced by the liquid flow. In this three-part series, we will focus on three major elements that can affect liquid flow: the pump rate, the capillary action, and the tubing.
Read MoreTags: mercury analysis, QuickTrace
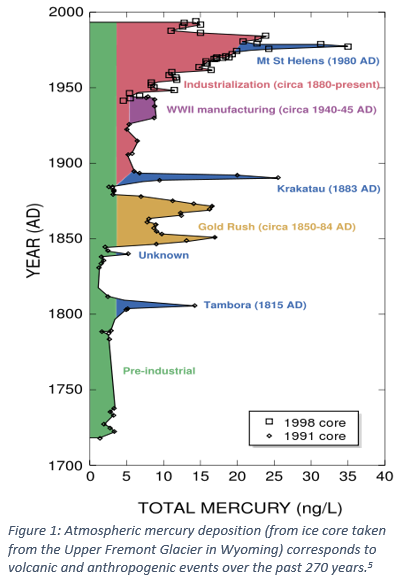
A lot of focus is put on anthropogenic (human-caused) releases of mercury into the environment, but let’s take a closer look into a powerful, natural source of mercury: volcanoes! We’ll explore why volcanic eruptions can increase global mercury levels, how we measure mercury released from past major eruptions, and how those measurements inform our policies for the future. So, how does mercury move from fire to ice?
Read MoreTags: mercury, mercury analysis
Hey Again! Let’s talk about having some fun while saving some money — lots of money, as a matter of fact!
In the world of green this and green that, with electric vehicles, lawn equipment, and scooters, we want to do our part to make the planet a better place.
Read MoreTags: mercury analysis
“While the invading forces marched in the streets of Copenhagen, I was busy dissolving Laue’s and also James Franck’s [Nobel Prize] medals.”1
Let us travel back to the 1940s. The Second World War is sweeping across Europe. As Denmark is being occupied by German forces, George de Hevesy doesn’t need to use weapons to protect his colleagues. He relies on science!
Read MoreTags: mercury analysis