Direct Analysis by Thermal Decomposition
 Direct mercury analysis is a well-established analytical technique for mercury determination used by the academic, biological, clinical, environmental, food, and industrial communities that has been approved by organizations such as the EPA in Method 7473 and the ASTM in Methods D6722 and D7623. This analysis employs the technique of thermal decomposition (sample combustion), mercury concentration via gold amalgamation, and detection by cold vapor atomic absorption. The biggest advantage of direct mercury analysis is no sample preparation or chemical reduction is required. The total mercury is released as a vapor when the sample is combusted. Advantages of thermal decomposition include:
Direct mercury analysis is a well-established analytical technique for mercury determination used by the academic, biological, clinical, environmental, food, and industrial communities that has been approved by organizations such as the EPA in Method 7473 and the ASTM in Methods D6722 and D7623. This analysis employs the technique of thermal decomposition (sample combustion), mercury concentration via gold amalgamation, and detection by cold vapor atomic absorption. The biggest advantage of direct mercury analysis is no sample preparation or chemical reduction is required. The total mercury is released as a vapor when the sample is combusted. Advantages of thermal decomposition include:
- Contamination is minimized
- Samples are introduced directly into the instrument
- Loss of sample is minimized
- No sample pre-treatment means little chance for a loss of mercury or a poor digest recovery
- No hazardous liquid waste is generated
- No need for strong acids, oxidizers, or (expensive) reducing agents
 Teledyne Leeman Labs Hydra IIC Direct Analysis Combustion Mercury System
Teledyne Leeman Labs Hydra IIC Direct Analysis Combustion Mercury System
The Teledyne Leeman Labs Hydra IIC is a fully automated analyzer that measures mercury in solid, semi-solid, liquid, and air sample matrices directly without any acid digestion. It offers many benefits including:
- Matrix independence
- No need to matrix match standards and samples
- One calibration curve suitable for a variety of sample types
- Fast analysis times
- < 6 min per sample in standard combustion mode
- Ease of operation
- Built in autosampler
How the Hydra IIC Mercury Analyzer Works
The Hydra IIC combustion atomic absorption (CAA) mercury analyzer is an independent, stand-alone analyzer that uses atomic absorbance (AA) spectroscopy to obtain reliable quantitative data from simple to complex sample matrices. By using direct combustion combined with a proprietary catalyst, interfering compounds such as sulfur and nitrogen oxides are removed. The combustion process entails a continuous linear flow of carrier gas through the entire system, including both the decomposition and catalyst furnaces. Figure 2 shows the system components and processes of the Hydra IIC CAA mercury analyzer.
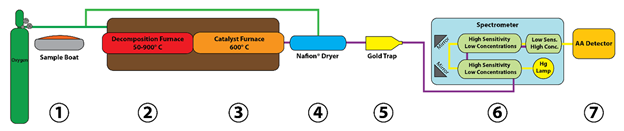
Figure 2: Principle of Operation
First a weighed sample (1) is introduced to a decomposition furnace (2) with oxygen (or air) flowing over the sample. The furnace temperature is then raised in two stages to first dry the sample, then to combust or decompose the sample. The gases resulting from the decomposition are carried through a catalyst furnace to remove halogens, nitrogen oxides, and sulfur oxides (3). The remaining combustion products, including elemental mercury (Hg0), are swept through a Nafion® dryer (4) and then to a gold trap (5) that captures the mercury while letting the other gases pass through. The gold trap is then heated to release the Hg0 into a carrier gas which transports it to the spectrometer (6). In the spectrometer, the gas is directed through two 5” high sensitivity (for low concentrations) optical cells, and then one 1” low-sensitivity (for high concentrations) optical cell. The light from the Hg lamp travels through the cells using a system of reflective mirrors. The transient signal is measured by the Atomic Absorption Detector (7). Waste gases exiting the system are chemically scrubbed with an activated carbon mercury trap or exhausted out of the lab at the end of the process.
The two peaks are integrated and reported against the best calibration of the two cells available. The use of two cells provides the best detection limit with a wider dynamic range than a detection limit provided by a single optical cell path length.
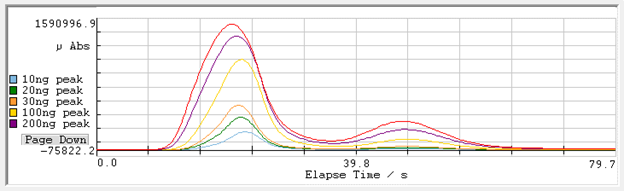
Figure 3: Calibration Standards Peak Profiles
Is CAA the Right Technique for Your Lab?
Selecting the right mercury analysis technique ultimately depends on your specific analytical needs. Answering a few simple questions could determine if CAA is right for your mercury determinations. Does your laboratory follow EPA 7473 or is your laboratory not bound by a regulatory method? Do you prefer not to digest samples? Are your samples mostly solid matrixes? If you answered yes, then the Teledyne Leeman Labs Hydra IIC will meet your needs and will be a sound, long-lasting investment.
Download your free Practical Guide to Selecting the Best Analytical Technique for your Mercury Measurement Needs by clicking on the button below
- S. EPA. 1998. "Method 7473 (SW-846): Mercury in Solids and Solutions by Thermal Decomposition, Amalgamation, and Atomic Absorption Spectrophotometry". https://www.epa.gov/sites/default/files/2015-07/documents/epa-7473.pdf
- 2020. “Method D6722-19: Standard Test Method for Total Mercury in Coal and Coal Combustion Residues by Direct Combustion Analysis”. https://www.astm.org/d6722-01.html
- 2020. “Method D7623-20: Standard Test Method for Total Mercury in Crude Oil Using Combustion-Gold Amalgamation and Cold Vapor Atomic Absorption Method”. https://www.astm.org/d7623-20.html
