Trace elemental analysis
 Teledyne Leeman Labs has specialized in trace elemental analysis for over 40 years. With initial success in Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES), also referred to as Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES), Teledyne Leeman Labs went on to develop innovative product lines. Those included branching out into various elemental analysis techniques, including DC Arc and Mercury Analysis. Our elemental analysis instruments can be found in laboratories across the world, providing service to a range of industries. From agriculture to aerospace and food to forensics, Teledyne Leeman Labs is dedicated to providing world-class instruments for trace elemental analysis.
Teledyne Leeman Labs has specialized in trace elemental analysis for over 40 years. With initial success in Inductively Coupled Plasma-Atomic Emission Spectroscopy (ICP-AES), also referred to as Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES), Teledyne Leeman Labs went on to develop innovative product lines. Those included branching out into various elemental analysis techniques, including DC Arc and Mercury Analysis. Our elemental analysis instruments can be found in laboratories across the world, providing service to a range of industries. From agriculture to aerospace and food to forensics, Teledyne Leeman Labs is dedicated to providing world-class instruments for trace elemental analysis.

Figure 1: Atomic Spectra for mercury (Hg), Copyright © Richard Pogge, All Rights Reserved
Atomic absorption spectroscopy
Atomic absorption spectroscopy (AAS) is one of the first commercially available elemental analysis techniques. This tried-and-true method remains a top choice for its simplicity and reliability. This analytical technique is used to determine how much of certain elements are in a sample. Atoms from each element absorb light at unique wavelengths. When this unique wavelength is applied to the sample, the atom absorbs the light energy. The electrons in the atom move from the ground state to an excited state and then relax back to the ground state. Using the Beer Lambert law, which describes the relationship between light absorption and concentration of an element, quantitative assessments of the sample can be made.
For example, the amount of energy it takes to move an electron from the ground state of a mercury (Hg) atom to the next excited level is equivalent to light at 253.7nm. In practice, a beam of light at this wavelength is passed through a carrier gas containing a mercury sample. The amount of light absorbed by the sample in the light path is measured and compared to the amount of light with no sample in the light path. The difference between the two amounts is the concentration of mercury in the sample. When samples are analyzed on an instrument which has been properly calibrated, the exact mercury concentration can be determined.
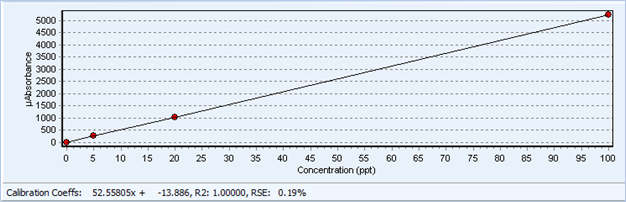
Figure 2: Calibration Curve from the QuickTrace M-7600
Mercury Analysis by CVAA Spectroscopy
With Teledyne Leeman Lab’s family of products, laboratories have their choice of atomic absorption spectrometers to fit a variety of testing requirements for the detection of mercury. Teledyne Leeman Labs offers two Cold Vapor Atomic Absorption Mercury Analysis Systems: the Hydra IIAA, an entry level system with detection limits less than 5.0 ng/L, and the QuickTrace M-7600, a dual beam system capable of ultra-trace detection limits less than 0.5 ng/L. Both instruments meet the requirements for various global mercury detection methods, including USEPA 245.1, USEPA 245.5, USEPA 245.6, USEPA 7470A, USEPA 7471B, EN 1483, EN 13806, and ISO 12846. The biggest difference between the two options is the usable range. The Hydra IIAA has a working range from low ng/L to mg/L levels, while the QuickTrace M-7600 is better suited for lower ranges, from sub ng/L to high µg/L.
For more detailed descriptions and comparisons of our CVAA systems, along with our other trace elemental analysis systems, please click below.

